Capitalizing on Sugar Feeding by Mosquitoes for Arbovirus Surveillance
Early detection of arbovirus transmission is critical for protecting humans and susceptible animals from infection with these dangerous pathogens. Arbovirus surveillance consists of concerted efforts by researchers or public health agencies to detect transmission of arboviruses by screening arbovirus hosts (vertebrate animals) or vectors (mosquitoes, ticks and other blood-feeding arthropods) for pathogens by various techniques. “Sentinel” surveillance consists of collecting and screening blood samples from wild or domestic animals (chickens, goats, dogs, wild songbirds or small mammals) for arbovirus exposure. This can give an important snapshot of virus transmission but is typically not useful for early warning purposes. Detecting arboviruses in the vectors provides a timely measure of current arbovirus transmission, allowing vector control districts the opportunity to intervene with control measures. Collecting vector species and screening them for arboviruses is time consuming and requires much technical expertise. Advances in arbovirus surveillance that increase efficiency and reduce complexity are needed.
Ongoing projects associated with advancing arbovirus surveillance include development of novel trapping methods as well as collaborations to develop push-button diagnostics. Central to this thrust are collective methods that take advantage of sugar-feeding by mosquitoes. Mosquitoes must feed upon plant sugars as a primary source of energy. Infectious mosquitoes deposit pathogens in their saliva. Trapped mosquitoes are provided with a sugar source that collects and preserves pathogens. The sugar can later be tested for arboviruses. This method has the advantage of pooling together the saliva (and possibly pathogens) of hundreds or thousands of wild mosquitoes.
Relevant publications:
Burkett-Cadena, N.D., Gibson, J., Lauth, M., Stenn, T., Acevedo, C., Xue, R.D., McNelly, J., Northey, E., Hassan, H.K., Fulcher, A. and Bingham, A.M., 2016. Evaluation of the honey-card technique for detection of transmission of arboviruses in Florida and comparison with sentinel chicken seroconversion. Journal of medical entomology, 53(6), pp.1449-1457.
Glushakova, L.G., Alto, B.W., Kim, M.S., Wiggins, K., Eastmond, B., Moussatche, P., Burkett-Cadena, N.D. and Benner, S.A., 2018. Optimization of cationic (Q)-paper for detection of arboviruses in infected mosquitoes. Journal of virological methods.
Burkhalter, K.L., Wiggins, K., Burkett-Cadena, N. and Alto, B.W., 2018. Laboratory evaluation of commercially available platforms to detect West Nile and Zika viruses from honey cards. Journal of medical entomology, 55(3), pp.717-722.
Contact: Nathan Burkett-Cadena, Barry Alto
Fig 1. Methods for collecting saliva from virus infected mosquitoes. Females that were observed with blue in their crop (inset) and their associated honey cards were removed for virus testing.
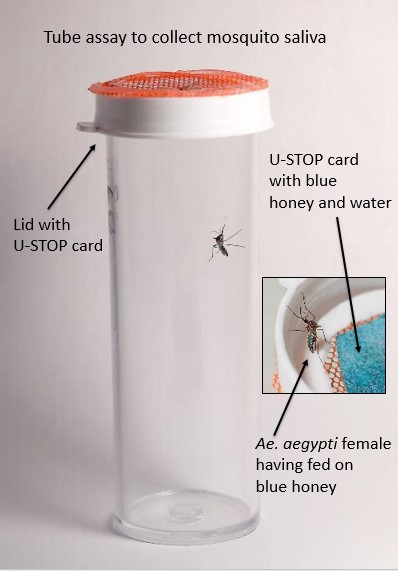
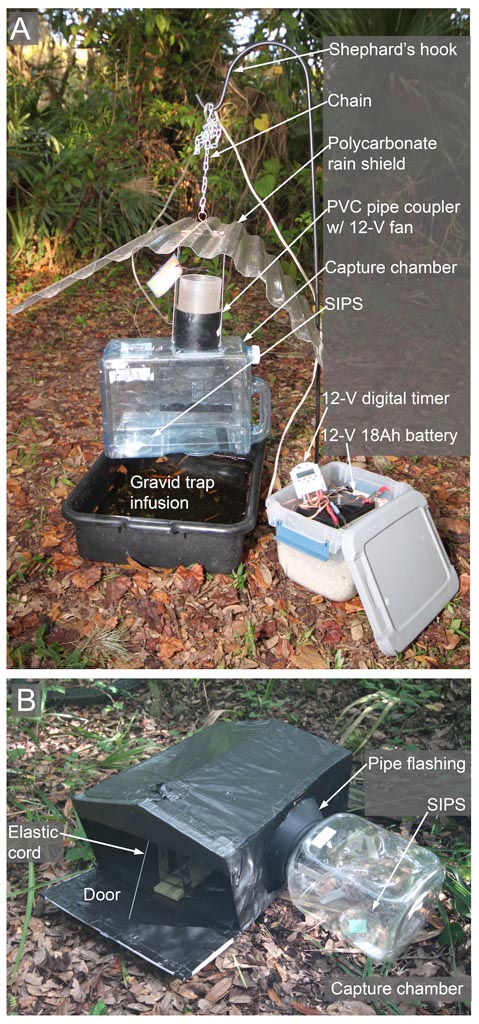
Fig 2. Traps modified for arbovirus surveillance. Gravid trap (A) and resting trap (B). SIPS: sugar impregnated preservative substrates. Figure modified from Burkett-Cadena et al. (2016).